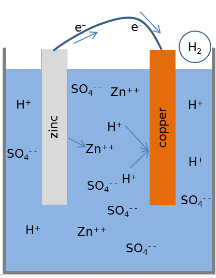
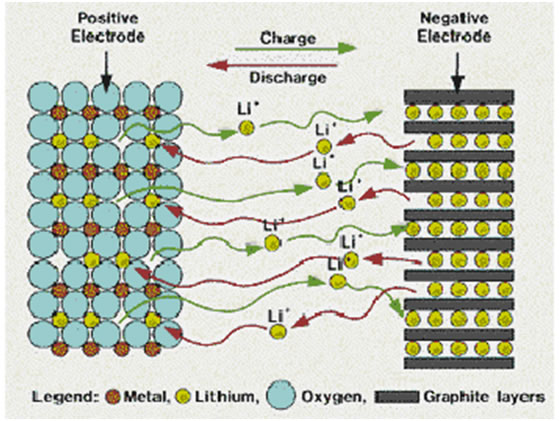
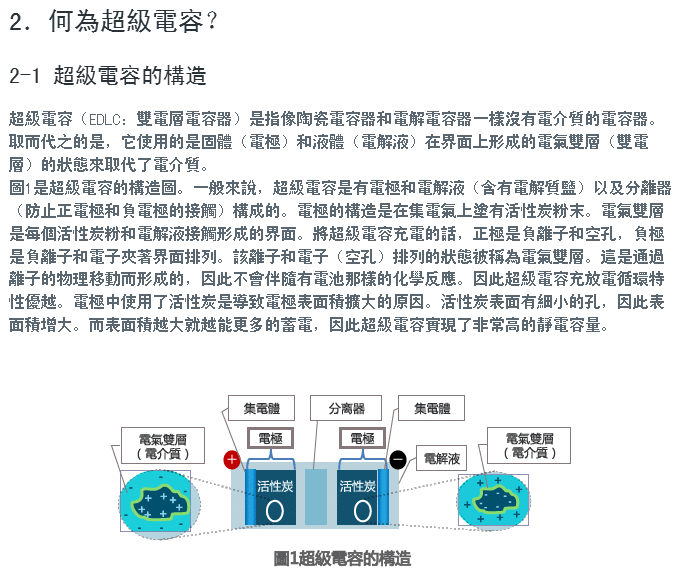
Lemon 電池
發生在 鋅電極 (氧化反應) :Zn → Zn2+ + 2e-
發生在 銅電極 (還原反應) :2H+ + 2e- → H2
氧化還原電位
https://en.wikipedia.org/wiki/Standard_electrode_potential_(data_page)
https://en.wikipedia.org/wiki/Reduction_potential
Al3+ + 3e- → Al(solid) : -1.662
乾電池
鹼性電池
平端 (陰極) Zn → ZnO
凸端 (陽極) MnO2 → MnOOH
http://boson4.phys.tku.edu.tw/science_works/Page178_Batteries_01.jpg
水銀